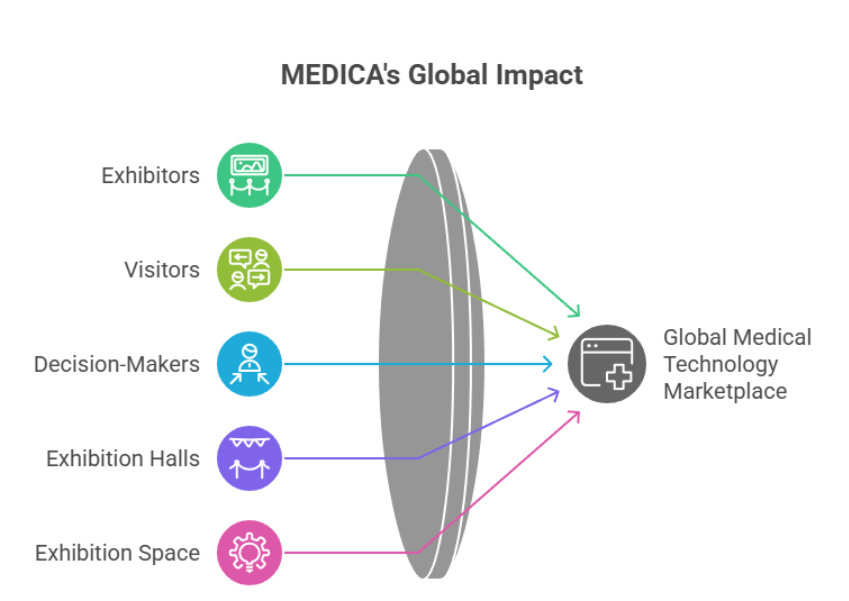
Unparalleled Global Scale and Relevance
MEDICA isn’t just a trade show—it’s the world’s largest global marketplace for medical technology, uniting the entire healthcare value chain in one location. The numbers are impressive:
Global Participation:
- More than 5,000 exhibitors from approximately 70 countries
- Around 80,000 professional visitors from over 150 nations
- 82% of visitors are key decision-makers (C-level, directors, managers)
- 17 exhibition halls dedicated to all areas of medical technology
- More than 140,000 m² of exhibition space
International Reach
- Presence of the largest players in the global market
- Representatives from regulatory agencies: FDA, Health Sciences Authority, Medical Products Agency, Health Products Regulatory Authority, and Anvisa
- Networking with decision-makers from hospitals, distributors, and governments
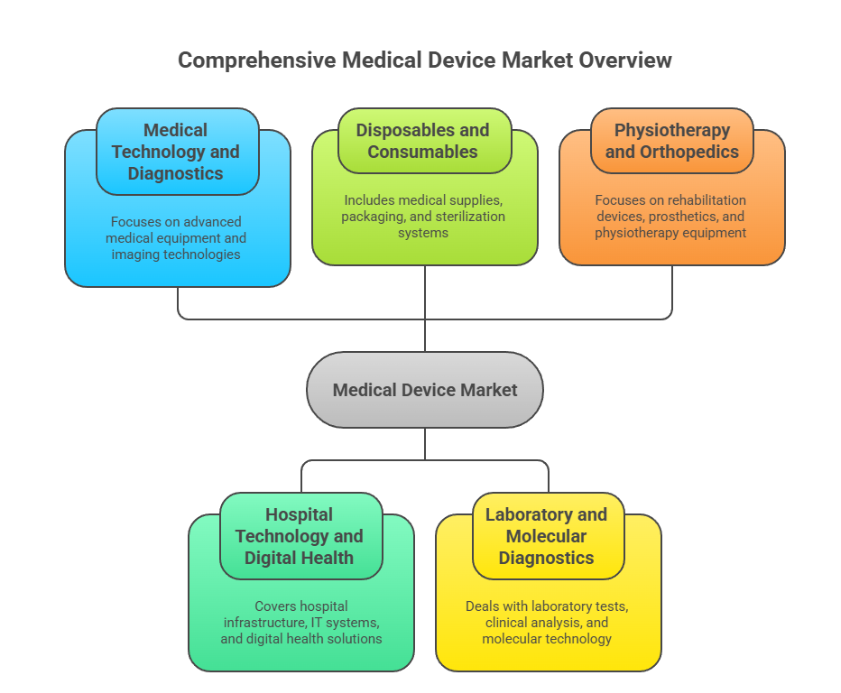
Comprehensive Technological Scope
MEDICA covers all segments of the medical device market, offering a 360° view of the sector:
5 Definitive Reasons Your Company Can’t Afford to Miss MEDICA 2025
1. Expansion into International Markets and Strategic Networking
Direct Access to Global Buyers
MEDICA brings together the world’s leading buyers and decision-makers. In four days, you can:
- Find qualified distributors in dozens of countries
- Negotiate representation and distribution contracts
- Establish joint ventures and strategic alliances
- Connect with international hospitals and healthcare networks
For Brazilian companies, MEDICA is the most efficient gateway to:
- European Market: Access to the 27 countries of the European Union
- American Market: Connection with distributors who already have FDA registration
- Asian Market: Networking with manufacturers and distributors from China, India, Korea, and Japan
- African and Middle Eastern Markets: Opportunities in emerging markets
High-Level Networking
Unlike regional trade fairs, MEDICA exclusively attracts qualified professionals:
- C-level executives from major corporations
- Purchasing directors from hospital networks
- Medical device specialists
- International regulatory authorities
- Investors and funds specializing in healthtech
Practical result: A 30-minute meeting at MEDICA can generate opportunities that would take months to establish through other means.
2. Competitive Intelligence and Global Trend Analysis
Complete Market View in One Place
MEDICA offers a unique opportunity for competitive benchmarking:
Competitive Analysis:
- Identify the main players in each segment
- Analyze price positioning and value proposition
- Evaluate technological differentiators
- Understand marketing and distribution strategies
Market Gap Identification:
- Discover under-explored niches
- Identify unmet needs
- Evaluate the viability of new products
- Validate innovation hypotheses
Technological Trends for 2025–2026
Based on previous editions and confirmations for 2025, the main themes will be:
Artificial Intelligence and Machine Learning:
- AI-assisted diagnostic algorithms
- Predictive health systems
- Automation of clinical processes
- AI regulation by the FDA and the European MDR
Software as a Medical Device (SaMD):
- Expanding telemedicine platforms
- Chronic disease monitoring apps
- Medical image analysis software
- Specific regulatory requirements for SaMD
Sustainability and ESG:
- Eco-friendly and biodegradable devices
- Circular economy in healthcare
- Carbon footprint reduction
- Sustainable packaging
Wearables and IoMT (Internet of Medical Things):
- Continuous monitoring wearable devices
- Integration with hospital systems
- Identifier software for data management
- Cybersecurity in connected devices
3D Printing and Personalization:
- Personalized prosthetics
- Anatomical models for surgical planning
- Bioprinting and tissue engineering
3. Specialized Workshops and Conferences
MEDICA offers more than 300 parallel events, including:
- MEDICA TECH FORUM: Presentations on disruptive innovations
- MEDICA CONNECTED HEALTHCARE FORUM: Digitalization and telemedicine
- MEDICA LABMED FORUM: Laboratories and diagnostics
- Regulatory workshops: Practical sessions on FDA, MDR, and GMP
Benefit for your company: Regulatory updates that would cost thousands of dollars in specialized consulting, available for free during the event.
4. Global Partnership and Distribution Opportunities
Find the Ideal Partner for Every Market
MEDICA facilitates strategic connections that drive growth:
For Manufacturers:
- Regional distributors with local expertise
- Commercial representatives established in target markets
- OEMs (Original Equipment Manufacturers) for customization
- Consultants in medical device consulting for regulatory compliance
For Distributors:
- Innovative manufacturers seeking partners
- Exclusive products to expand your portfolio
- Reliable suppliers with GMP certifications
- Access to laboratory developed tests and emerging technologies
For Service Providers:
- International clients who need FDA registration
- Companies seeking MDSAP certification
- Manufacturers needing identifier software
- Opportunities for manufacturing outsourcing
Technological Partnerships
MEDICA is also a hub for collaborative innovation:
- Joint development agreements with universities and research centers
- Licensing of patented technologies
- Partnerships for clinical validation of new products
- Technology transfer agreements
Conclusion: The Smartest Investment for 2025
MEDICA isn’t just a trade fair—it’s the single most important strategic investment your company can make to grow in the global medical device market.
Recapping the 5 Reasons to Attend:
- International expansion and networking with 80,000 qualified professionals
- Competitive intelligence and access to the latest technological trends
- Regulatory updates directly from the sources (FDA, MDR, MDSAP, Anvisa)
- Strategic partnerships that drive exponential growth
- (The fifth reason, missing from the bullet points in the source text, likely refers to the “Comprehensive Technological Scope” or a related benefit mentioned earlier)
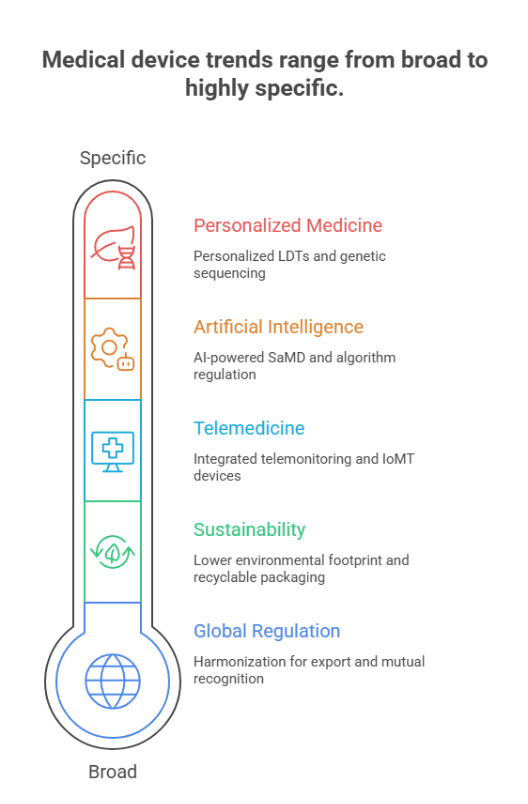
MEDICA 2025 promises to be the largest and most innovative edition in history, with a special focus on:
- Artificial Intelligence and SaMD
- Sustainability and ESG
- Telemedicine and digital health
- Global regulatory harmonization
- Personalized medicine
Find out more about BPO in RA!
*Budget for registration ownership transfer, Market Access Strategy, and BPO in RA services for your company: www.brisa.com.br



